
- About
- Patient Information
- Health Care Professionals
- Donate
REPORTING DEVICE COMPLICATIONS TO THE MHRA
In 2014 there were some concerns raised in Scotland about the safety of synthetic meshes for prolapse and incontinence surgery. The MHRA have a reporting system for medical devices similar to the “yellow card” drug adverse event reporting system. To date the number of reports to the MHRA from medical practitioners of adverse events is less than would be expected. The following is a guide to reporting device complications to the MHRA.
What is an adverse incident?
The MHRA define an adverse incident as "an event that causes, or has the potential to cause, unexpected or unwanted effects involving the safety of device users (including patients) or other persons." In other words any actual or potential adverse event which occurs when employing a device which affects the patient, healthcare professional or other user
What to report
Any adverse incident involving a device should be reported to the MHRA, including problems with the instructions for use or difficulties with deployment, especially if the incident has led, or might have led to:
- deterioration in health or permanent impairment of body structure or function
- the necessity for medical or surgical intervention (including implant revision)
- hospitalisation or prolongation of existing hospitalisation
- death, life-threatening illness or injury
For synthetic meshes for prolapse and incontinence this may include:
- Vaginal exposure
- Erosion into the urinary tract
- Erosion into the bowel or rectum
- Infection
- Pain
- Fistulae
- Mesh shrinkage
- Organ perforation
- Nerve or vascular injury
- Sexual difficulty
These may present to the original operating surgeon but may present later to other gynaecologists, urologists or colorectal surgeons.
Who should report
Adverse incidents can be reported by
- Clinicians and healthcare workers
- Patients and members of the public
- Device manufacturers
Role of medical device liaison officers in NHS trusts
NHS trusts and primary care trusts in England have all designated a member of staff as their medical device liaison officer (MDLO). Their role is to promote and coordinate adverse incident reporting and help to manage the dissemination of medical device alerts.
When a device related adverse event is reported to MHRA, the MDLO and/or risk manager should also be informed in line with local protocol.
Confidentiality and data protection
The MHRA does not require patient names or other identifying information in order to carry out an investigation. Healthcare staff reporting incidents should, therefore, ensure that such details are deleted or redacted from their reports, from accompanying attachments and from any subsequent correspondence.
The details required by the MHRA are specified on the report forms. The reporter's full contact details (name, post held etc.) are essential, as this allows the MHRA to contact the reporter to acknowledge receipt of the report and request any further information that may be needed.
How to report
The preferred and simplest way to report adverse incidents involving devices to the MHRA is via the on-line forms on the MHRA website (http://www.mhra.gov.uk). This also provides facilities for copying the incident through to MDLOs, risk managers or other local relevant staff in compliance with any good practice in local reporting policies. You may however also fax, email or post a downloadable form instead.
Each devolved administration has its own guidance on reporting adverse incidents, available on the respective websites.
Northern Ireland (external link)
Scotland (external link)
Wales (external link
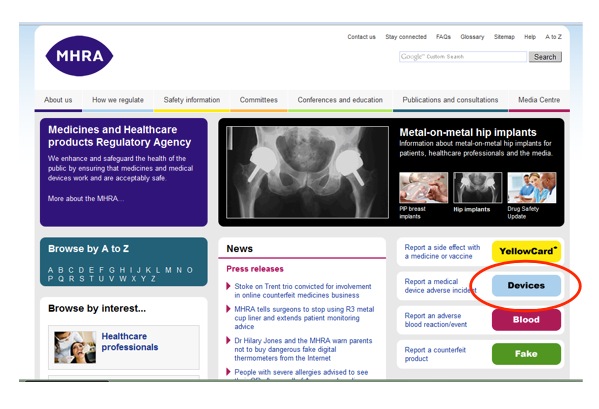

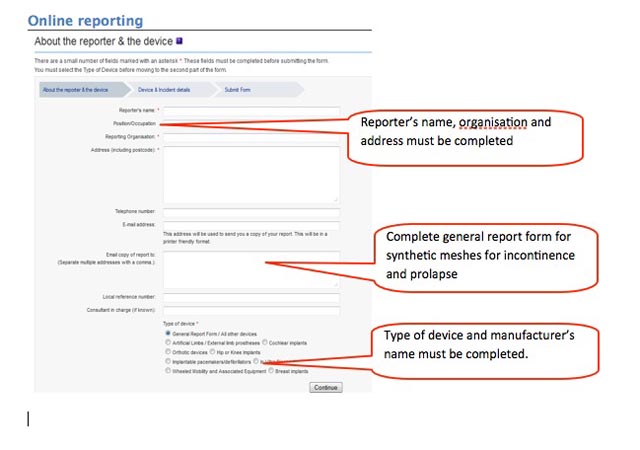
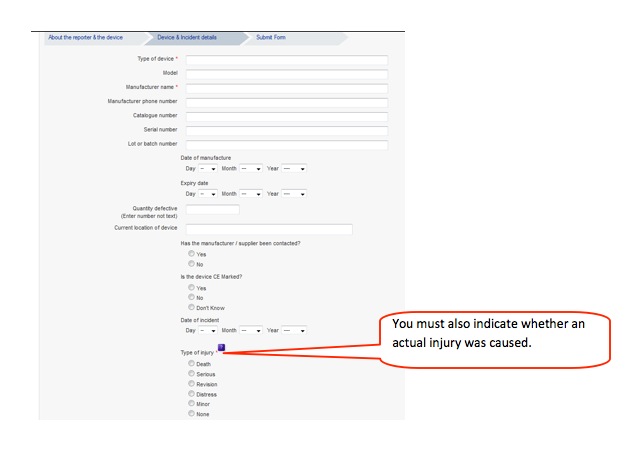
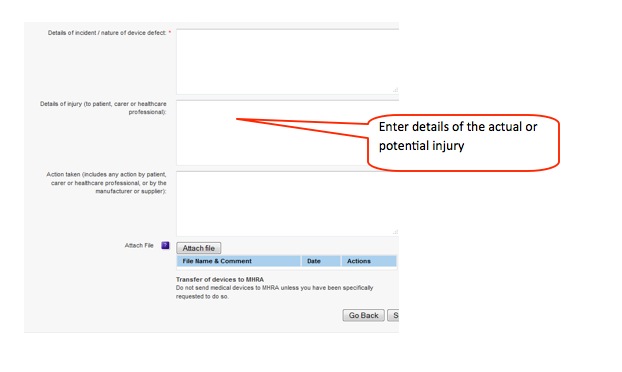
How to report an adverse incident report (England and Wales):
1. Go to MHRA website
2. Follow links to "Devices" then "Medical device adverse incident reporting forms"
3. Complete form, preferably online. The core information required is very simple:
- who you are and where you are reporting from;
- the name of the device and manufacturer;
- what happened and was there an injury.
4. If you include your own email address on the online form, MHRA will email you a pdf copy of your completed form.
5. Inform Trust medical devices liaison officer or equivalent in your Trust. You can do this electronically by also entering their email address on the online form.



